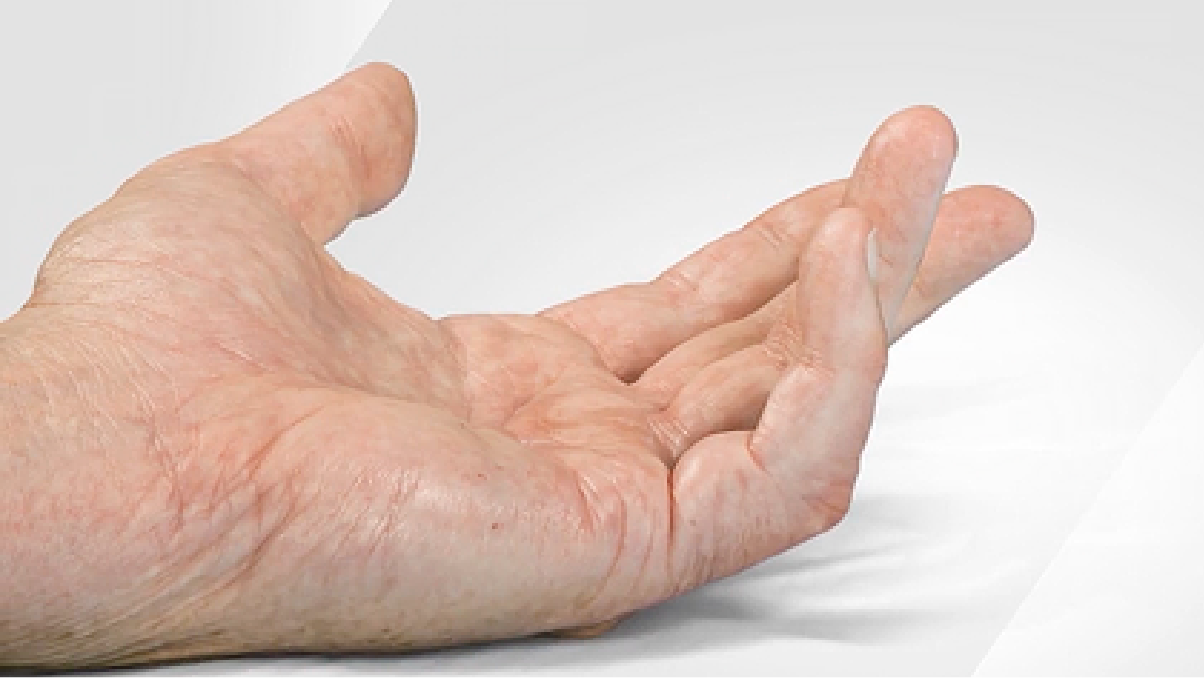
In clinical trials, XIAFLEX® was studied in 20°–80° contractures in the PIP joint6
ANTHONY Fifth finger 47° PIP contracture
- Range of motion limited to 42°
- Father and brother both diagnosed with Dupuytren’s contracture
- Significant progression in recent months
- 59 years old
- Pastry chef
- Enjoyed playing guitar before his contracture worsened

 Watch Dr Fanuele treat a similar contracture with XIAFLEX®
Watch Dr Fanuele treat a similar contracture with XIAFLEX®