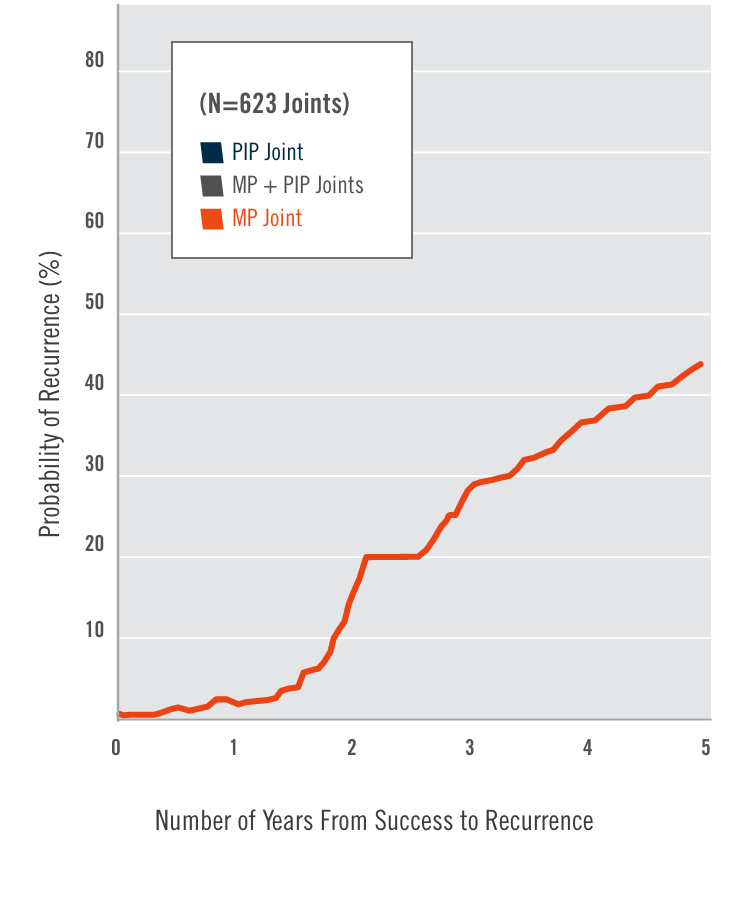
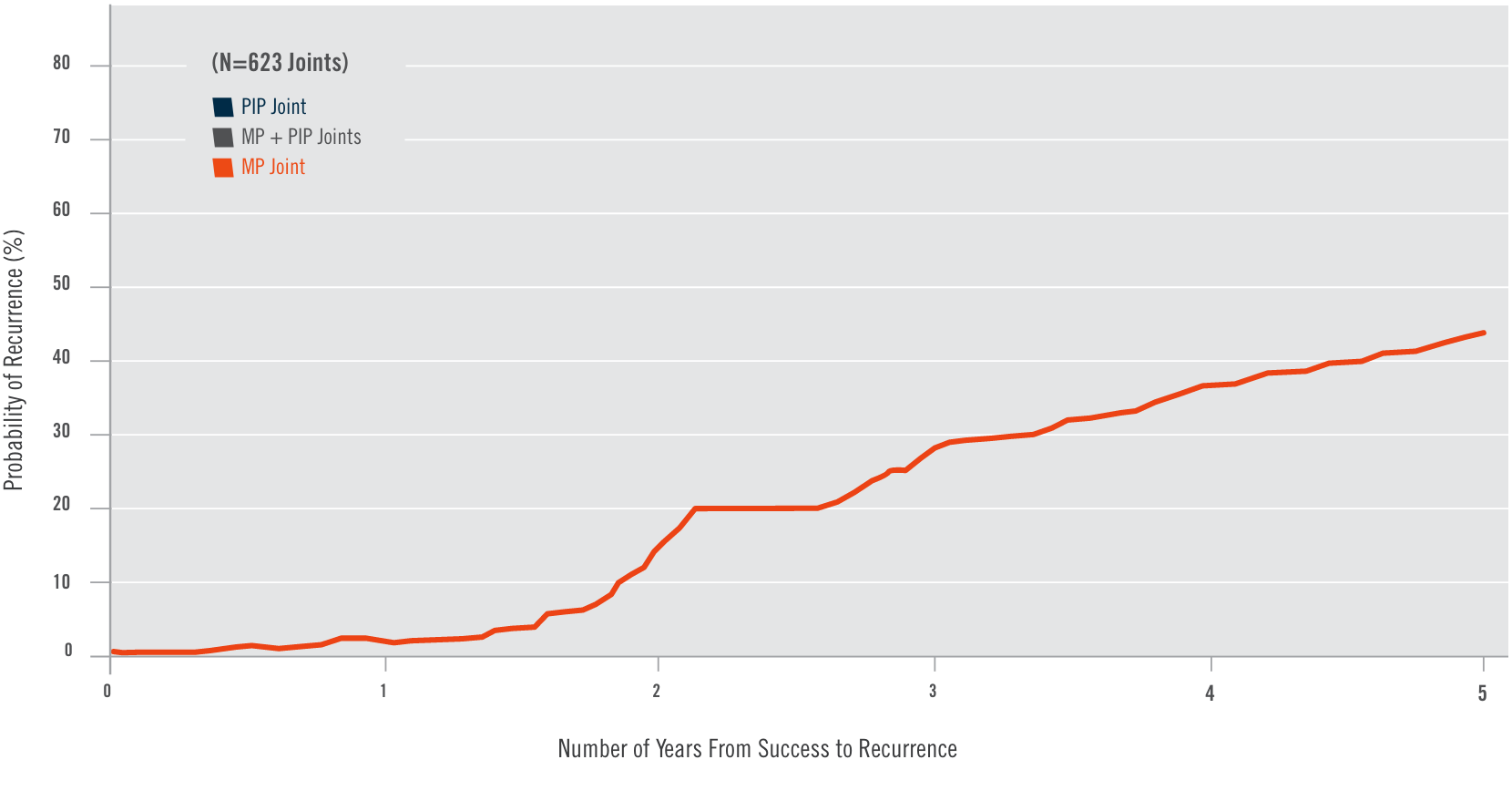
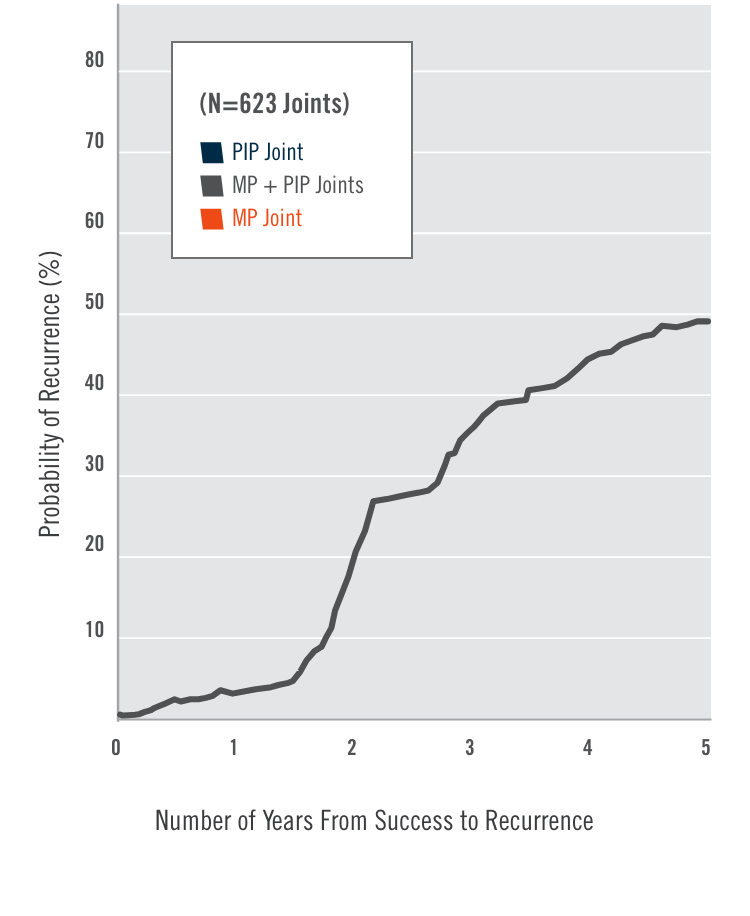
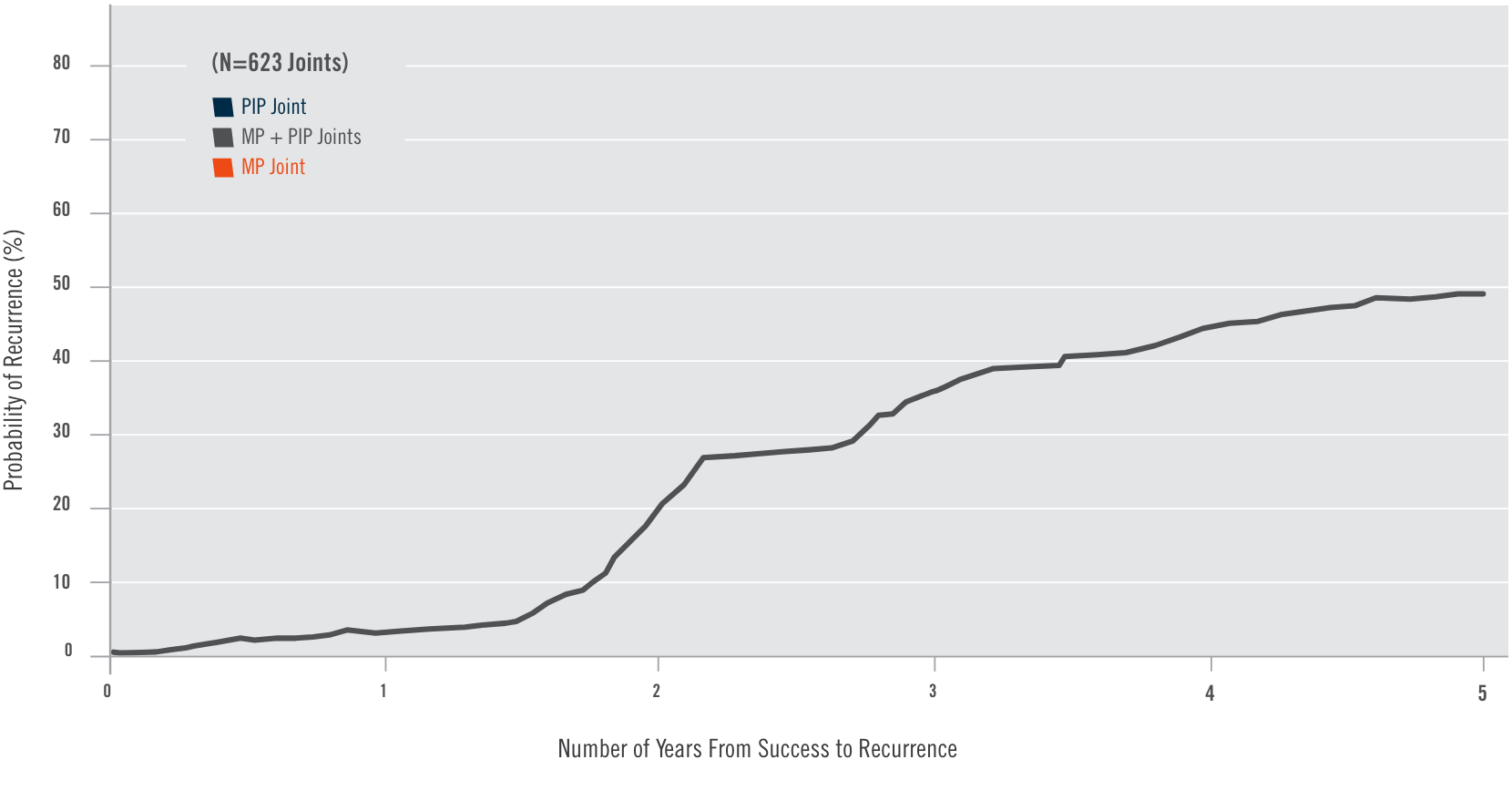
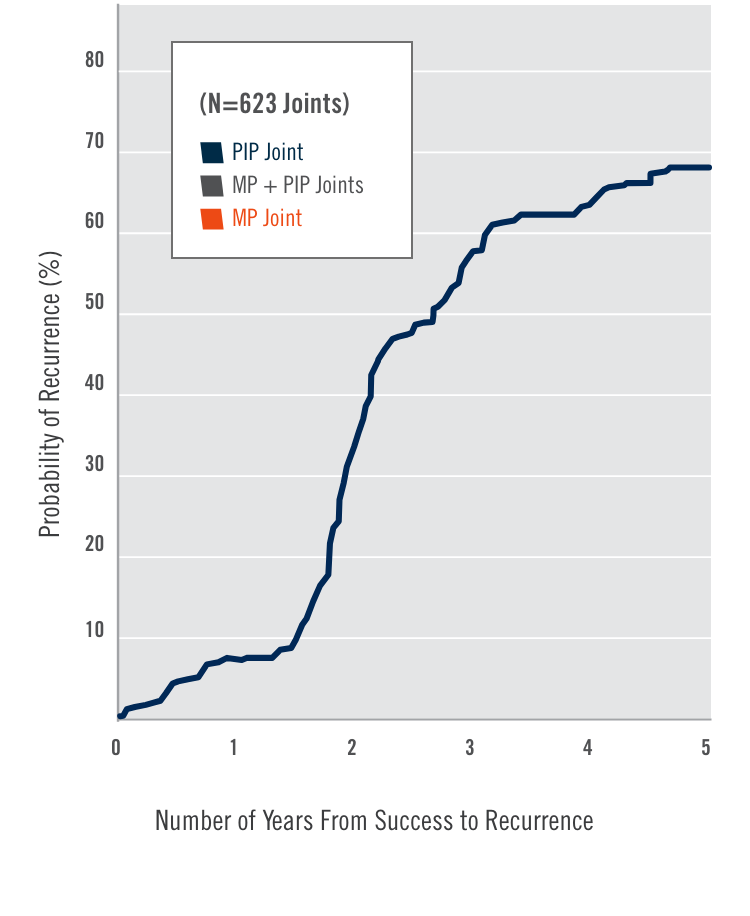
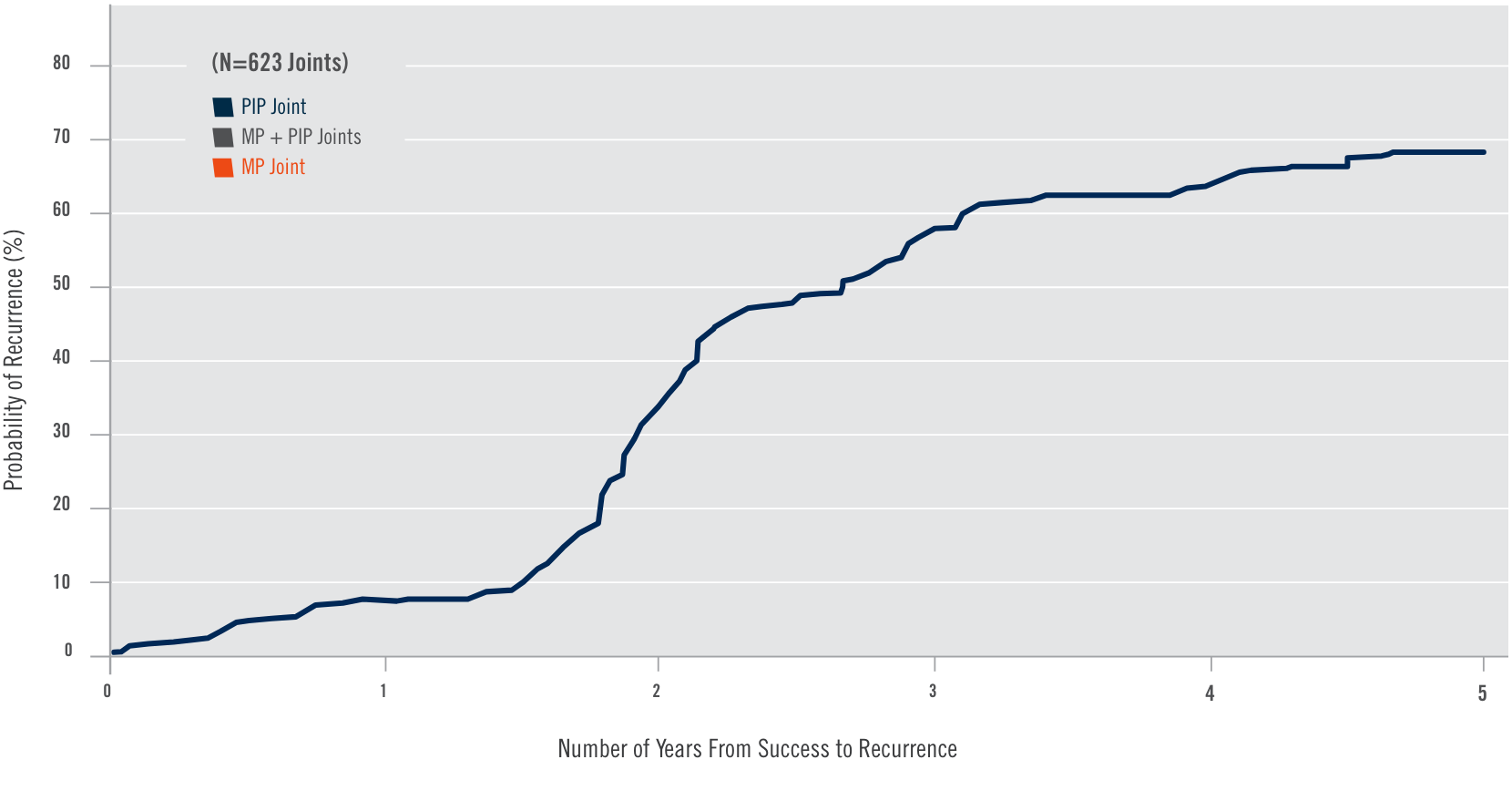
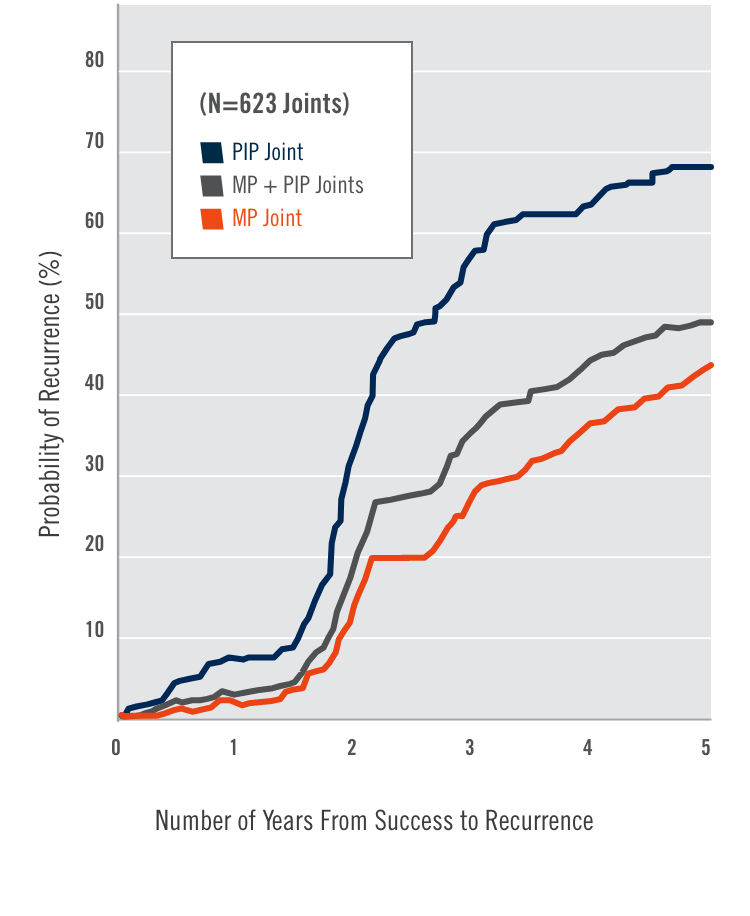
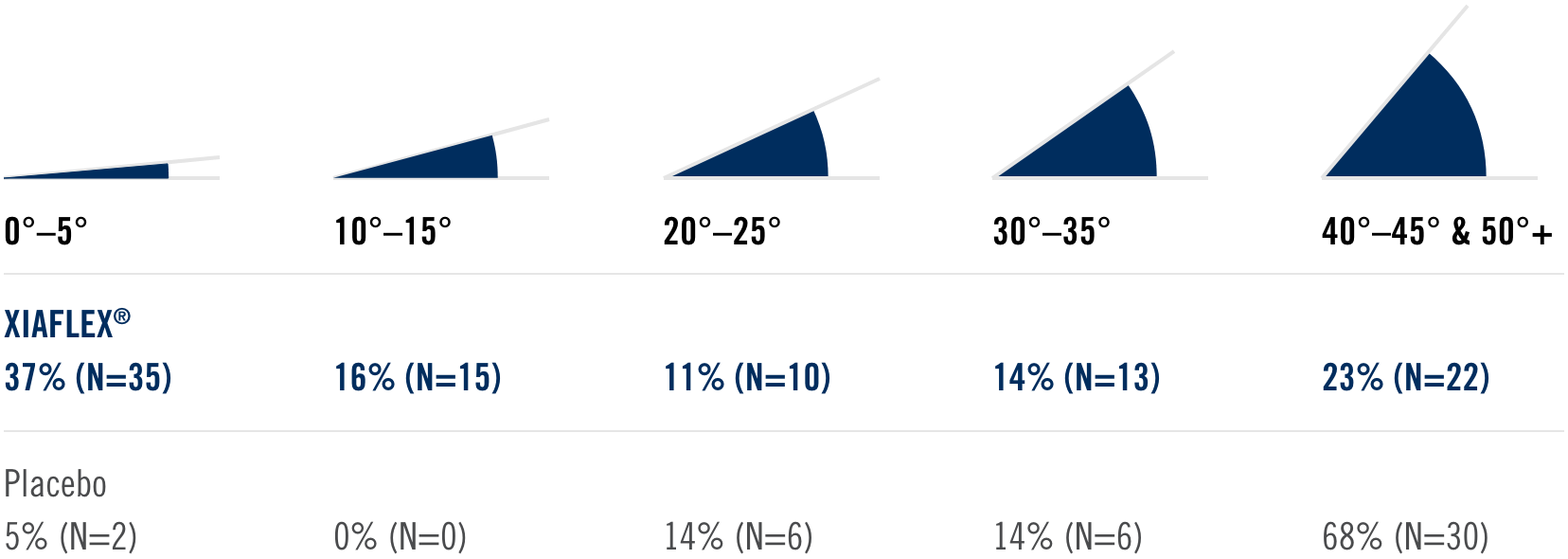CORD I and CORD II study design
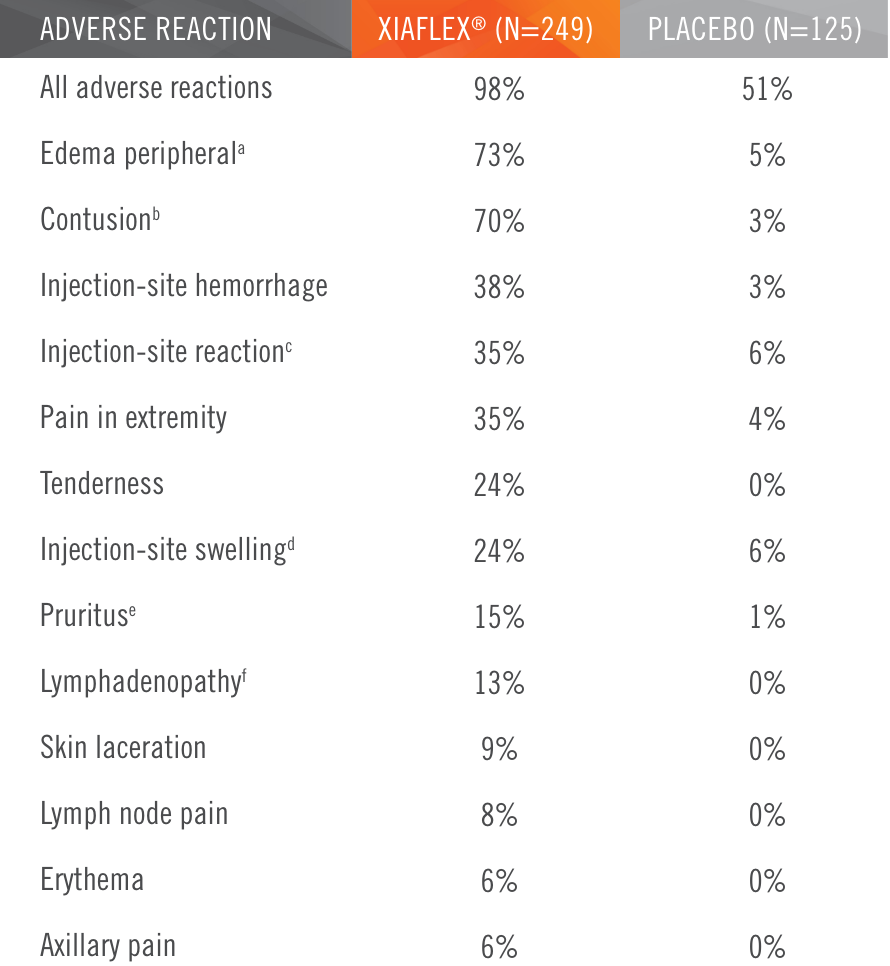
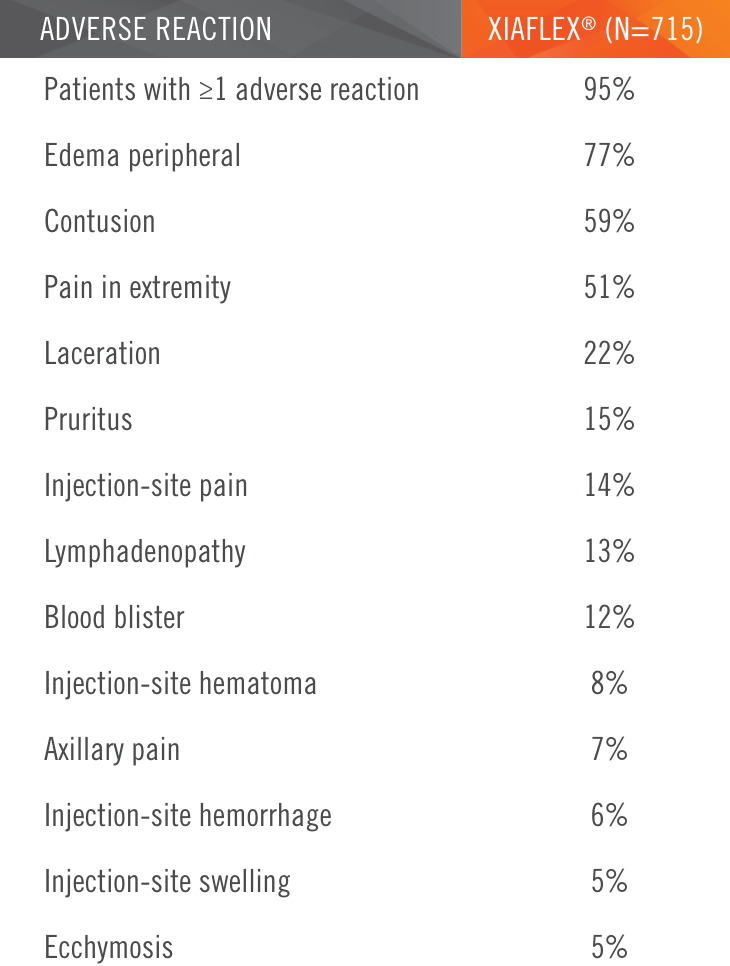
Two randomized, double-blind, placebo-controlled, multicenter pivotal studies (N=374)1
SINGLE JOINT (Study 1 and Study 2)
Design
Randomized, double-blind, placebo-controlled, multicenter pivotal studies (N=374)1
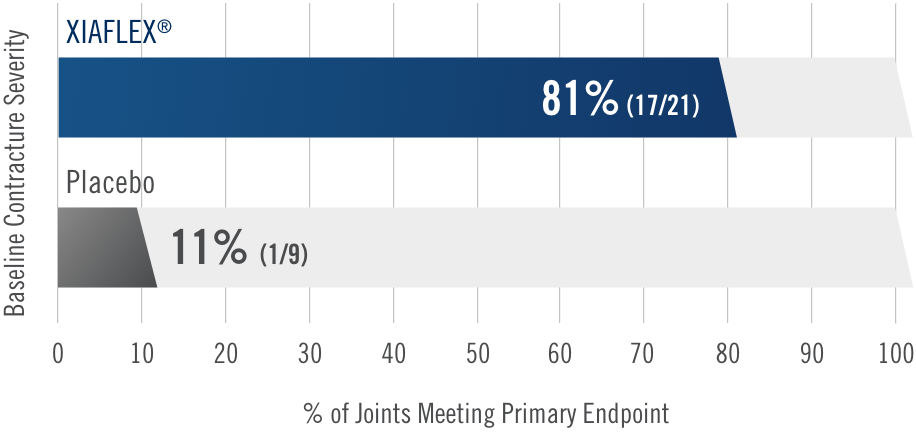
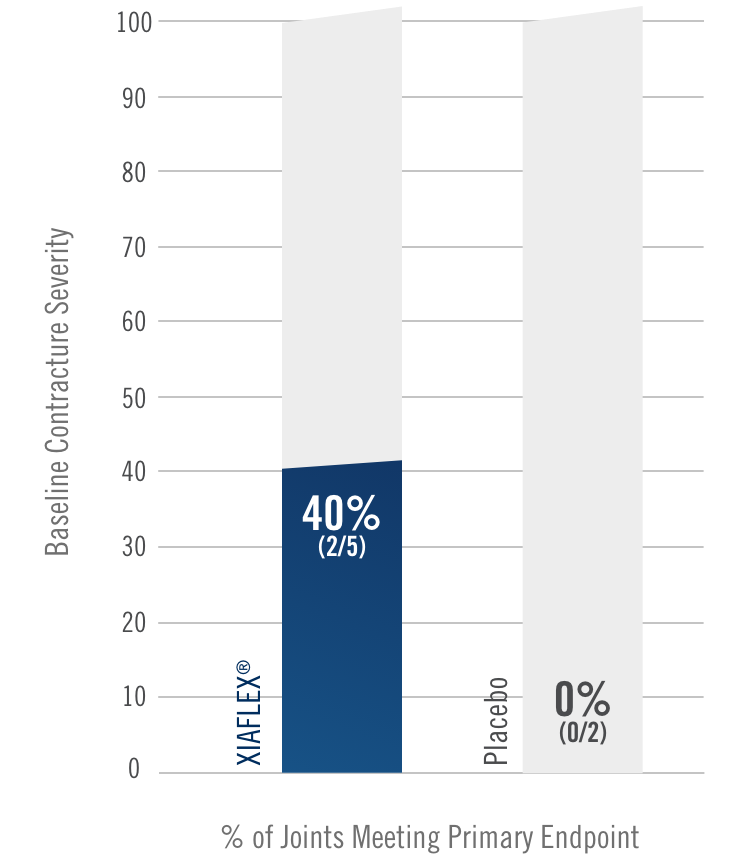
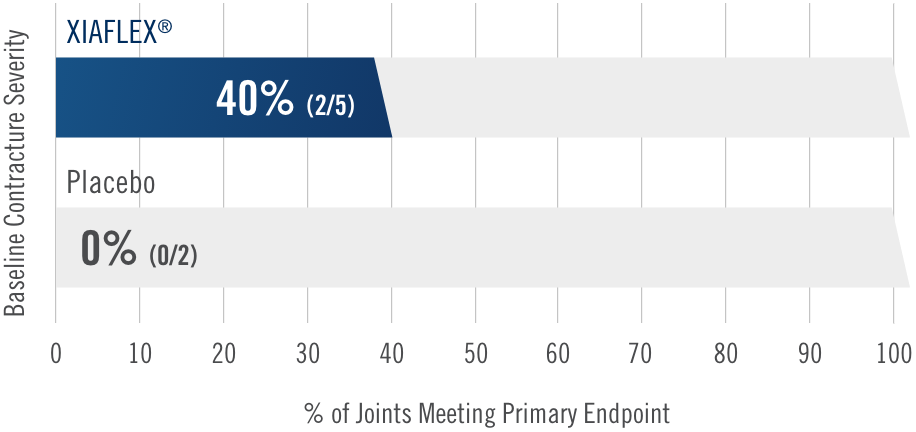
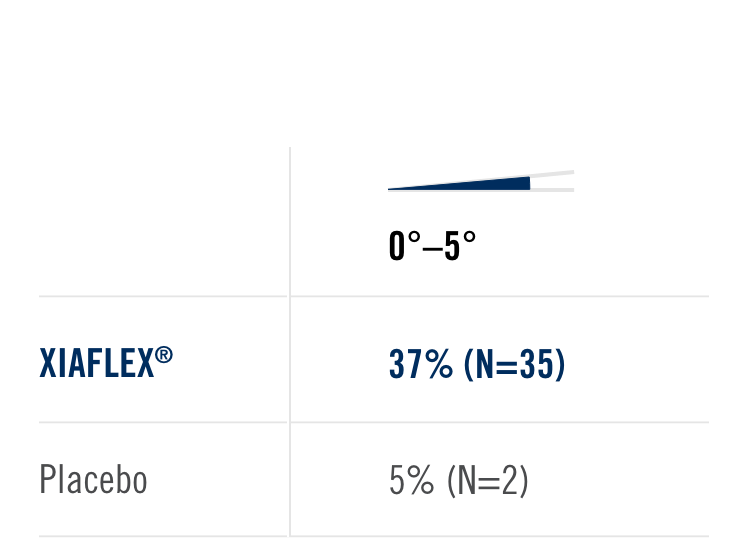
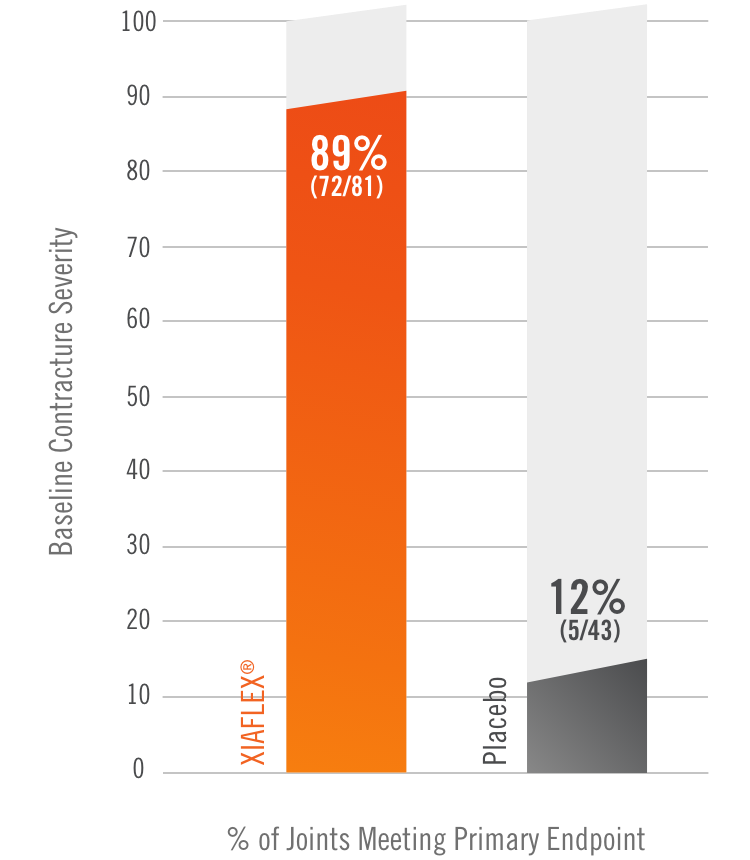
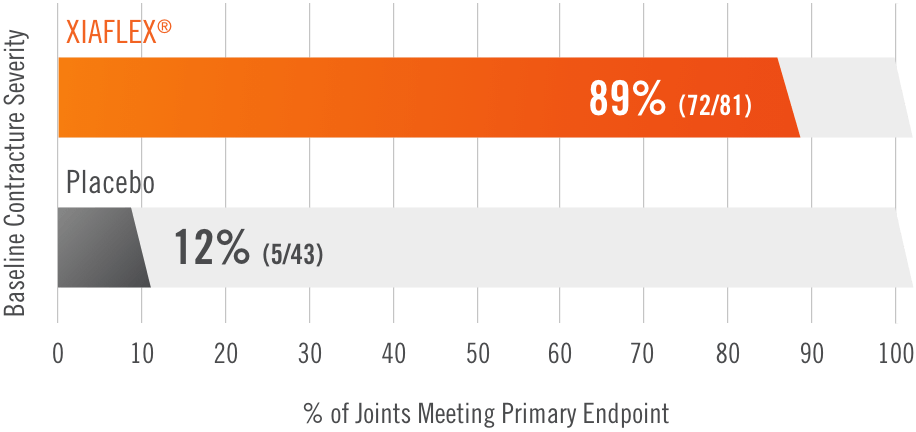
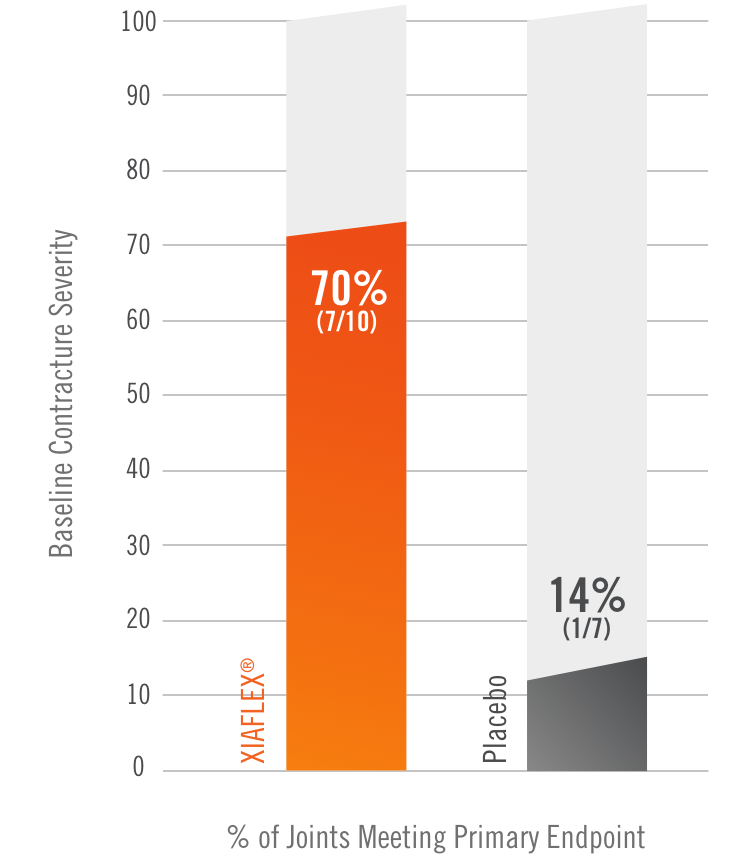
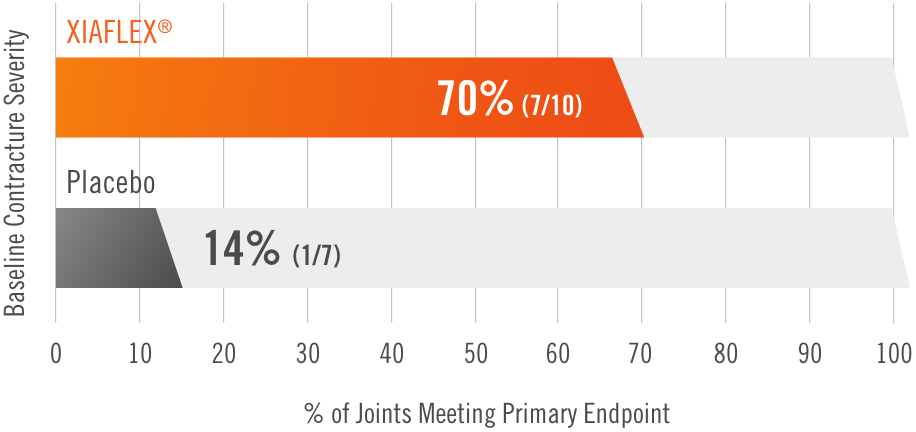
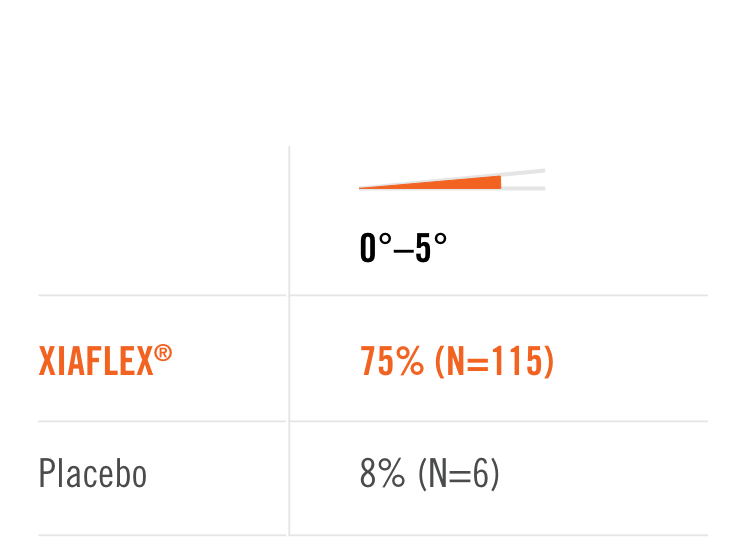
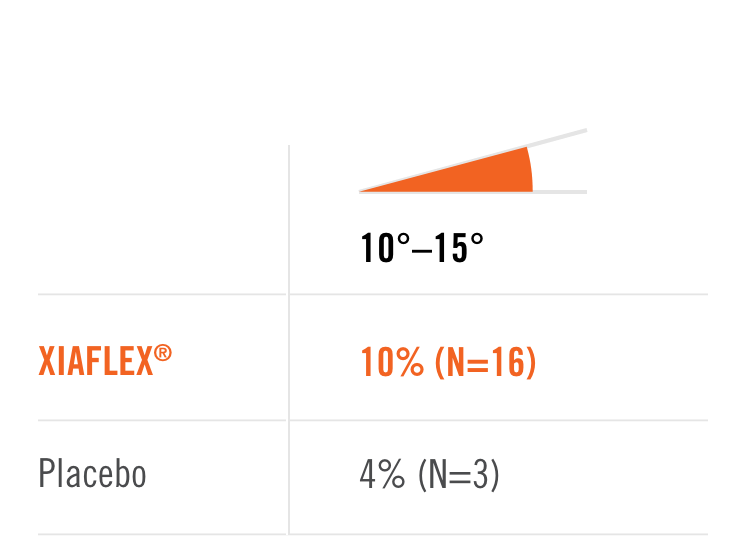
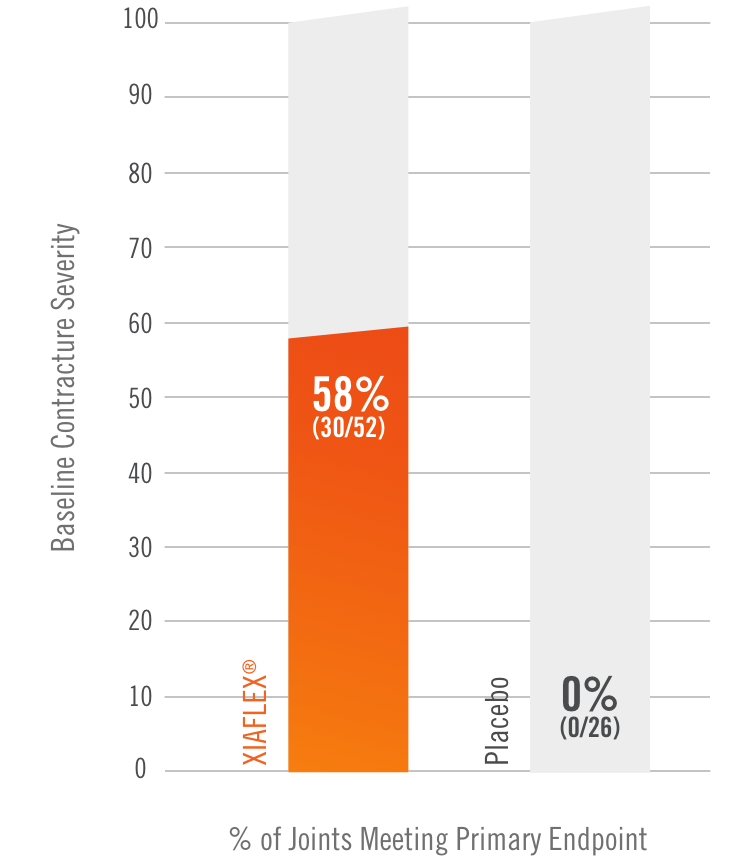
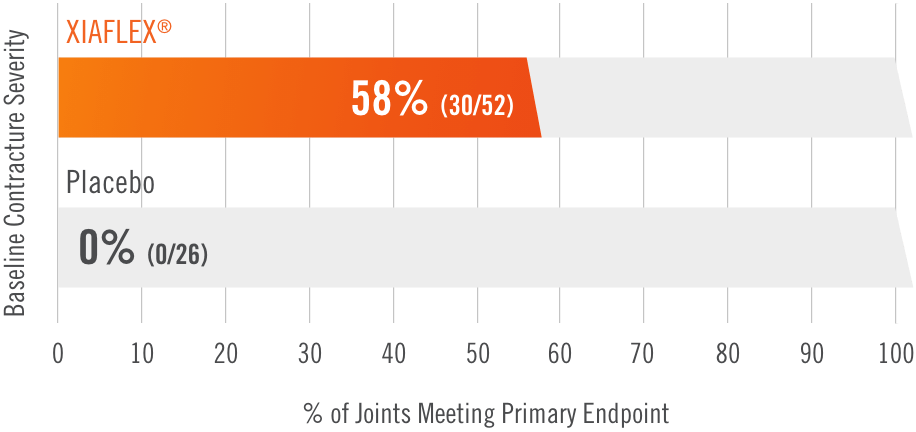
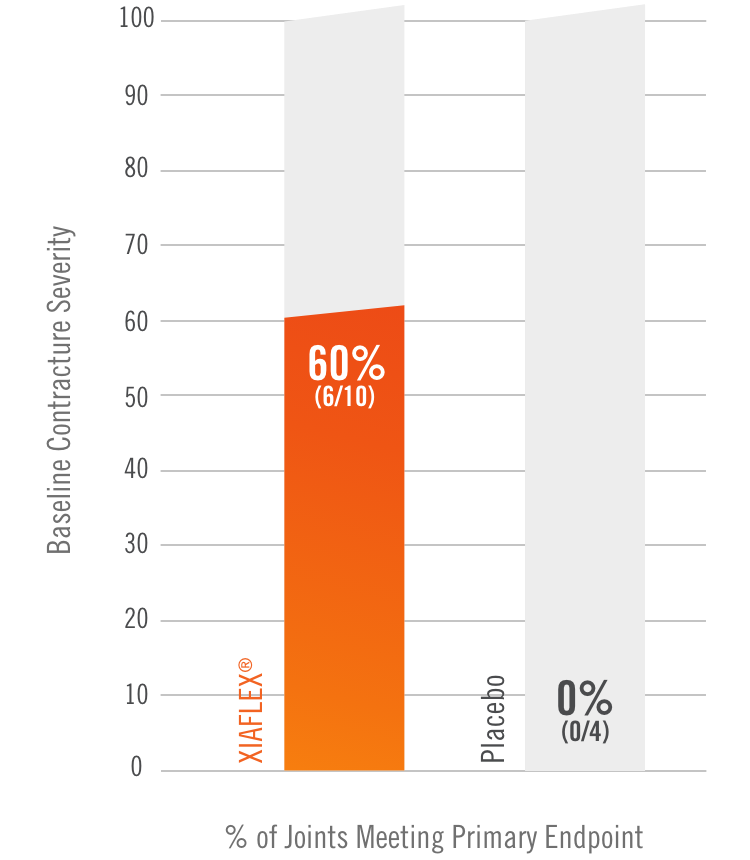
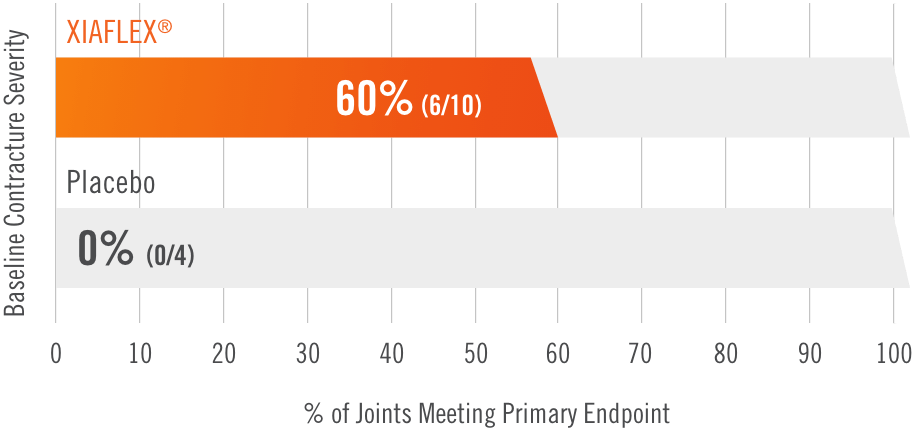
Primary endpoint1
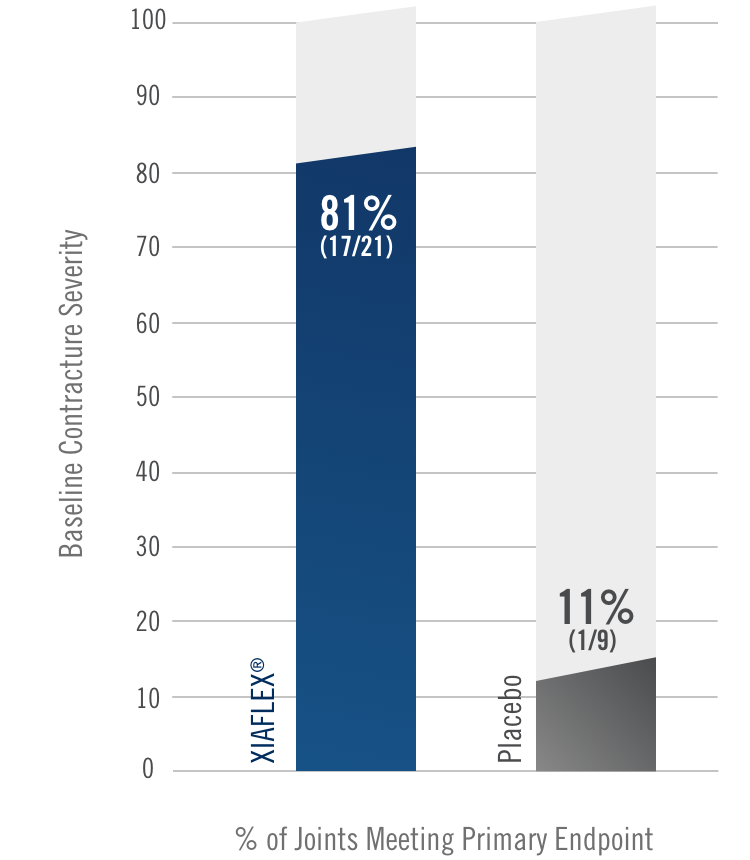
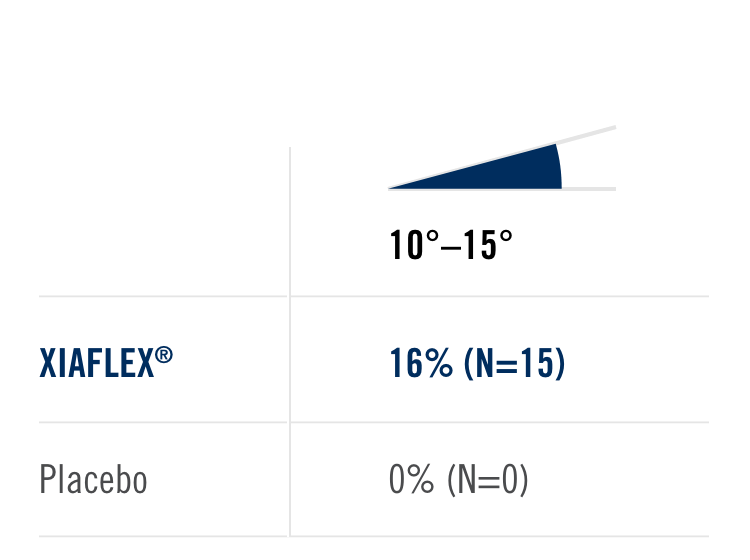
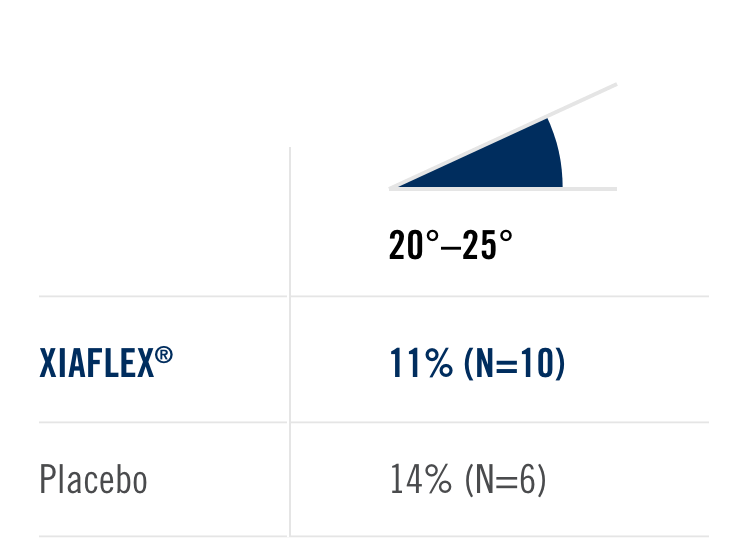
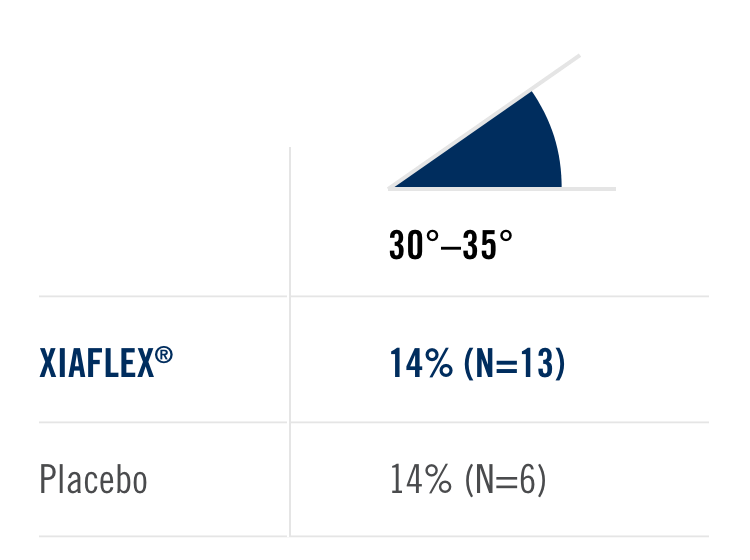
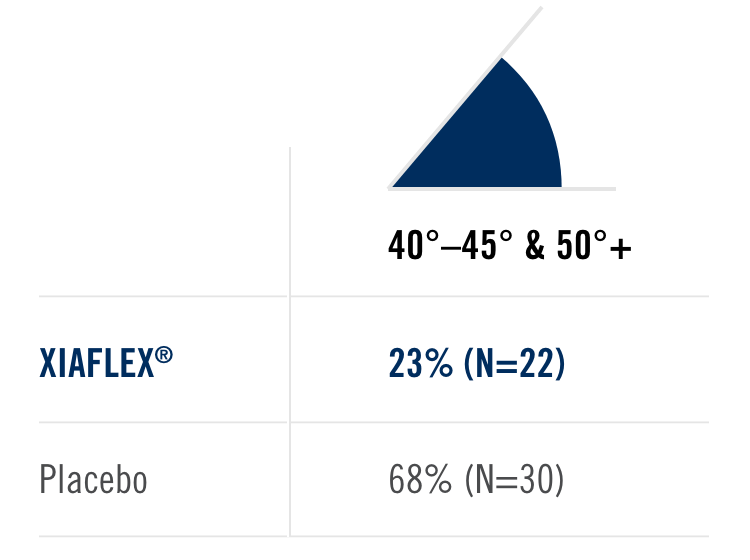
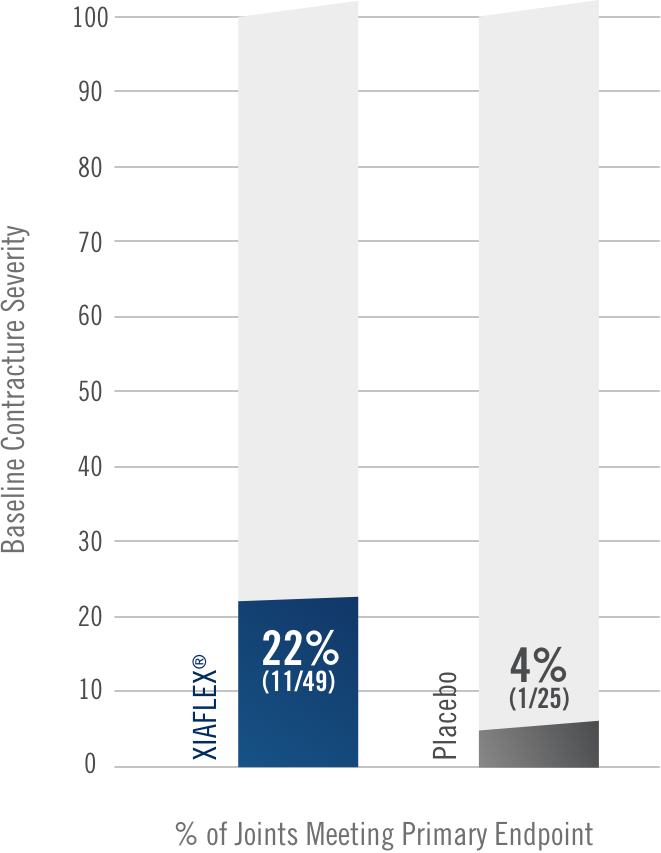
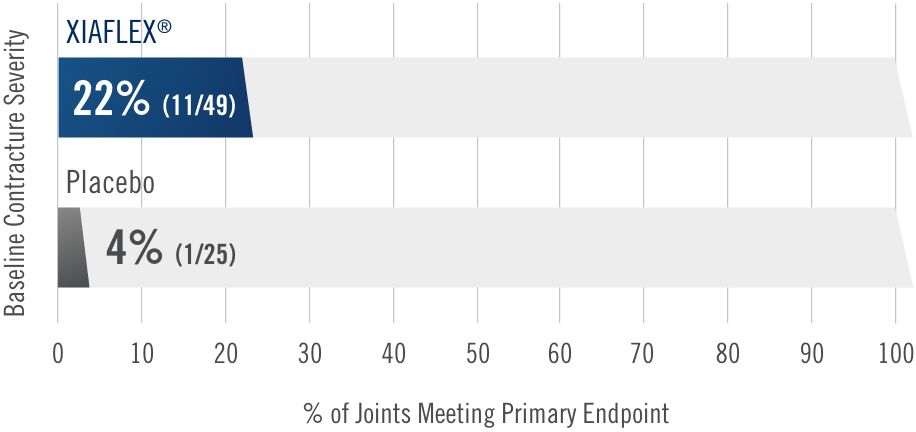
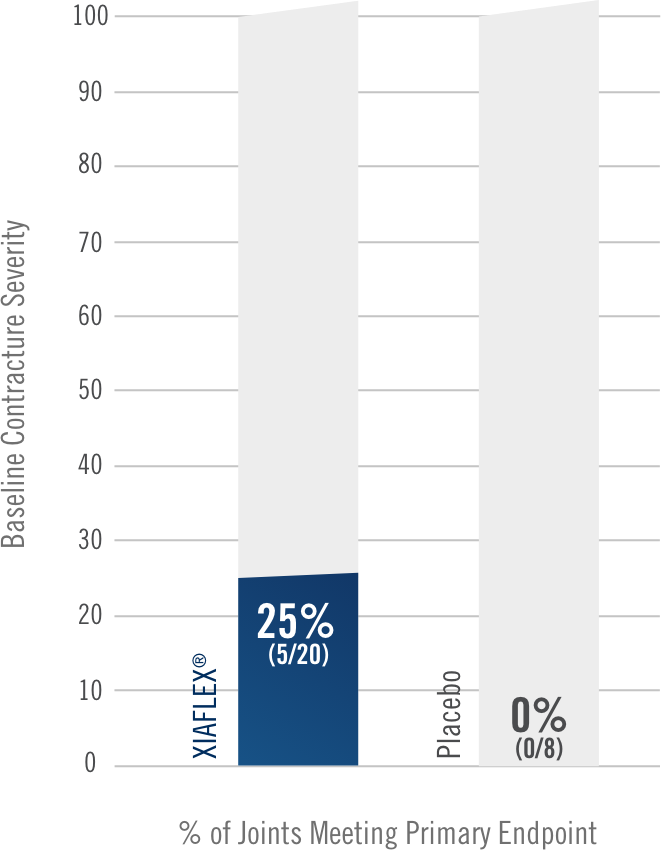
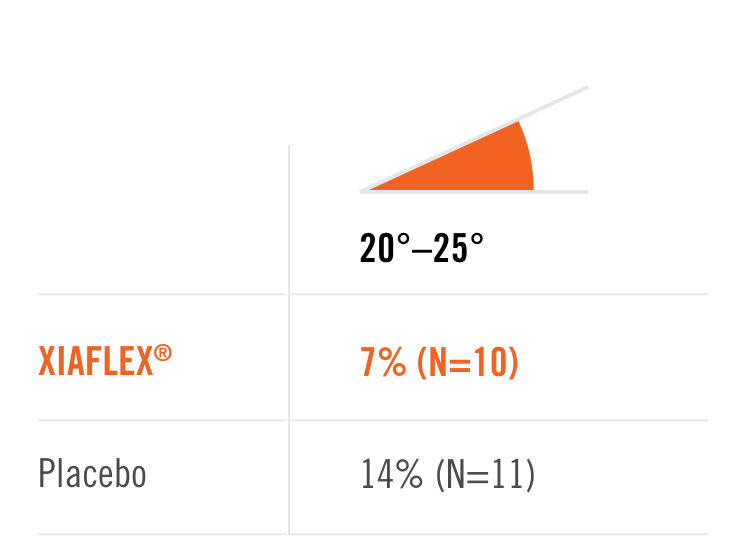
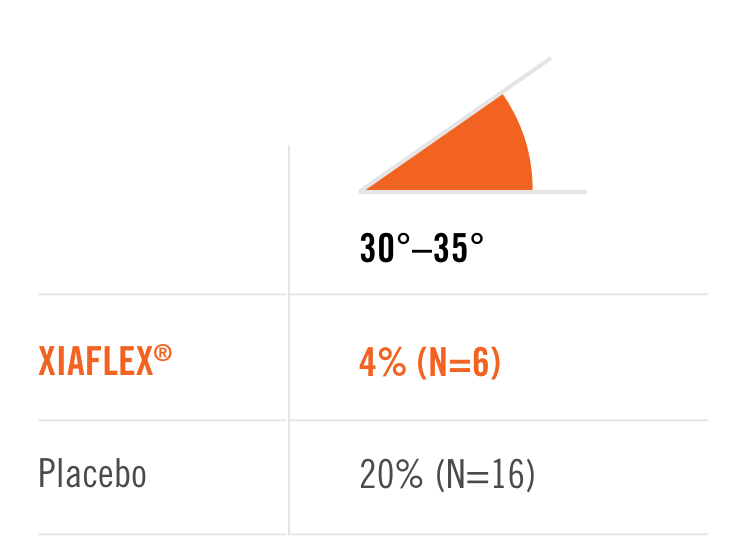
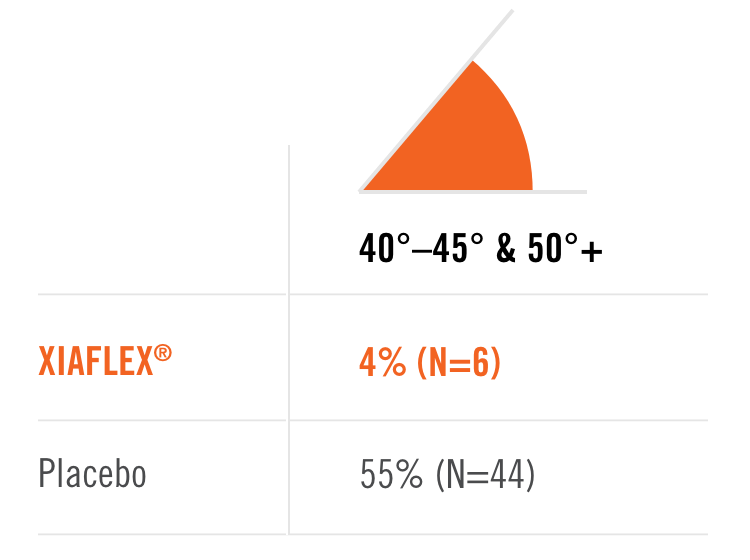
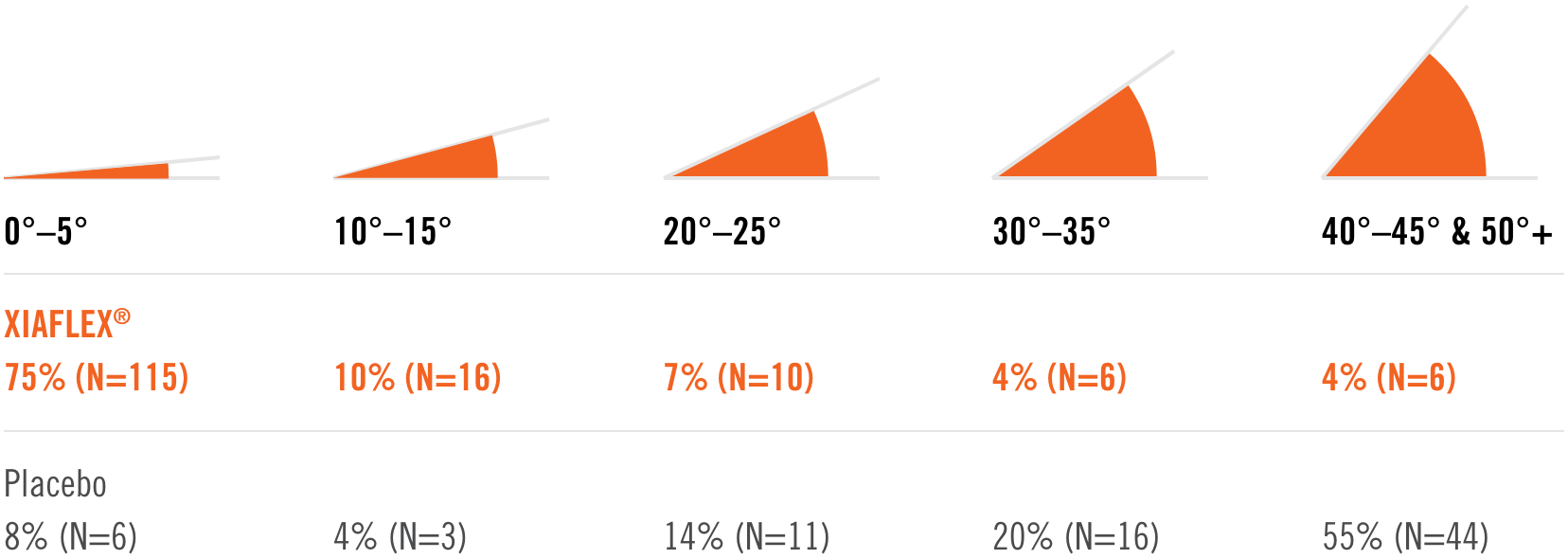
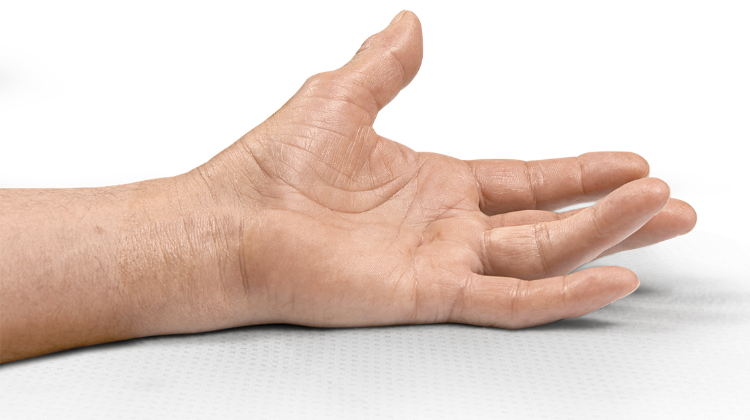
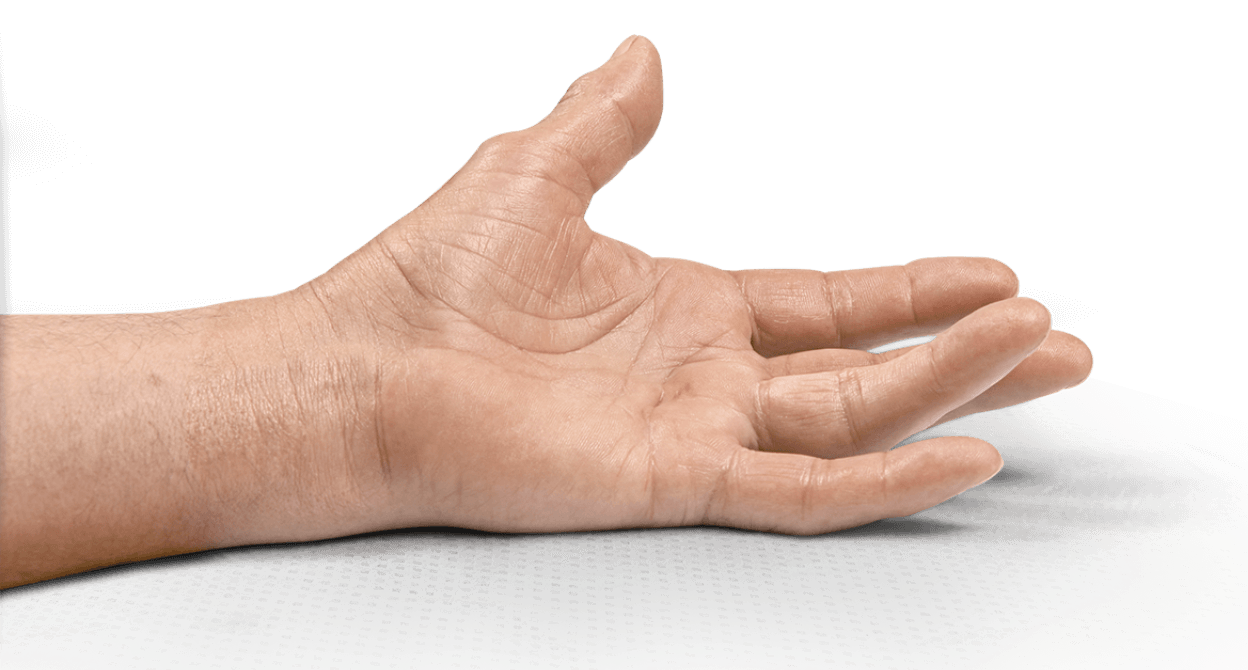
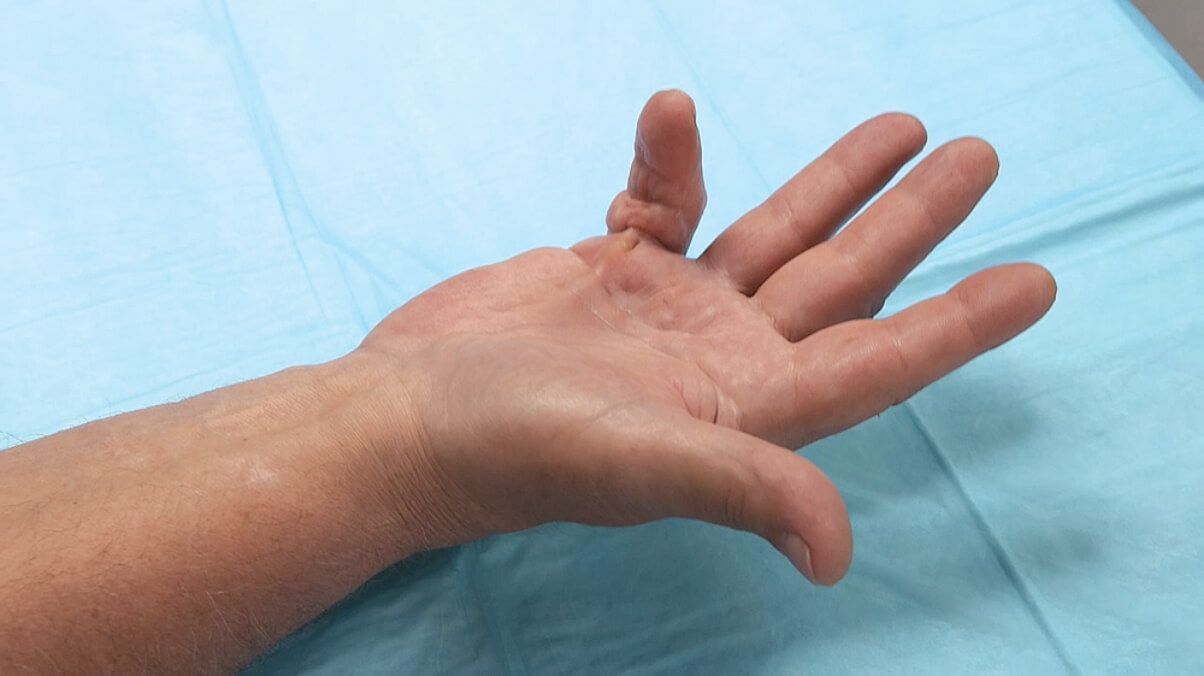
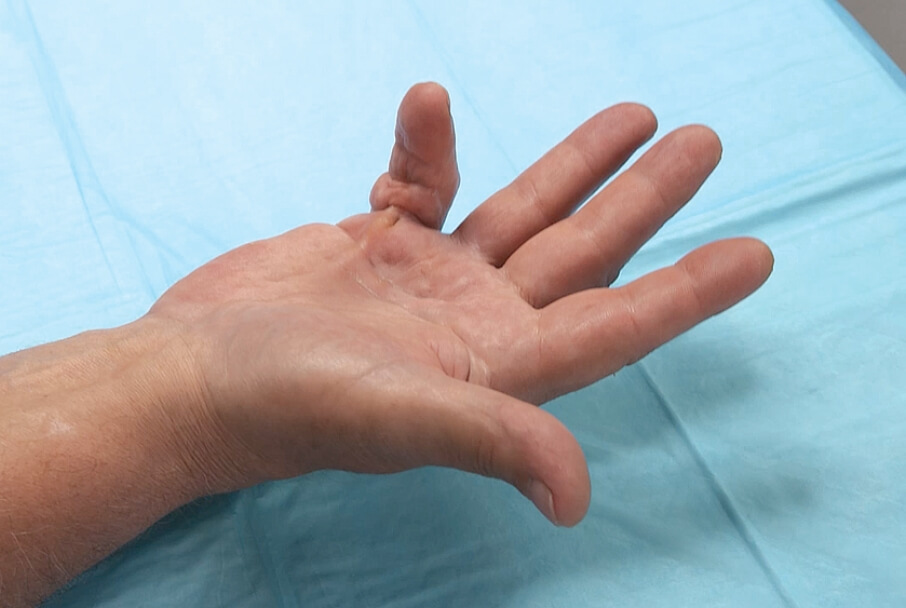
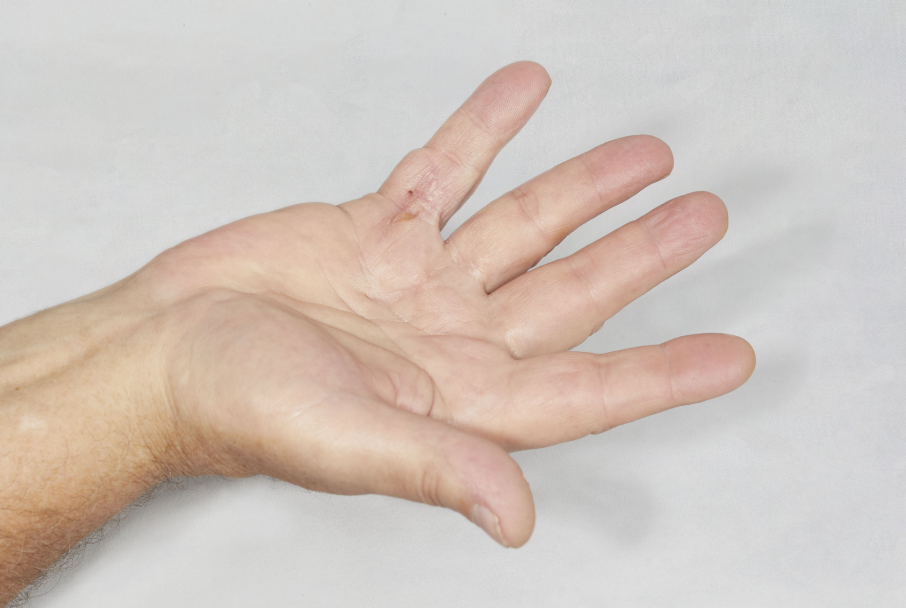
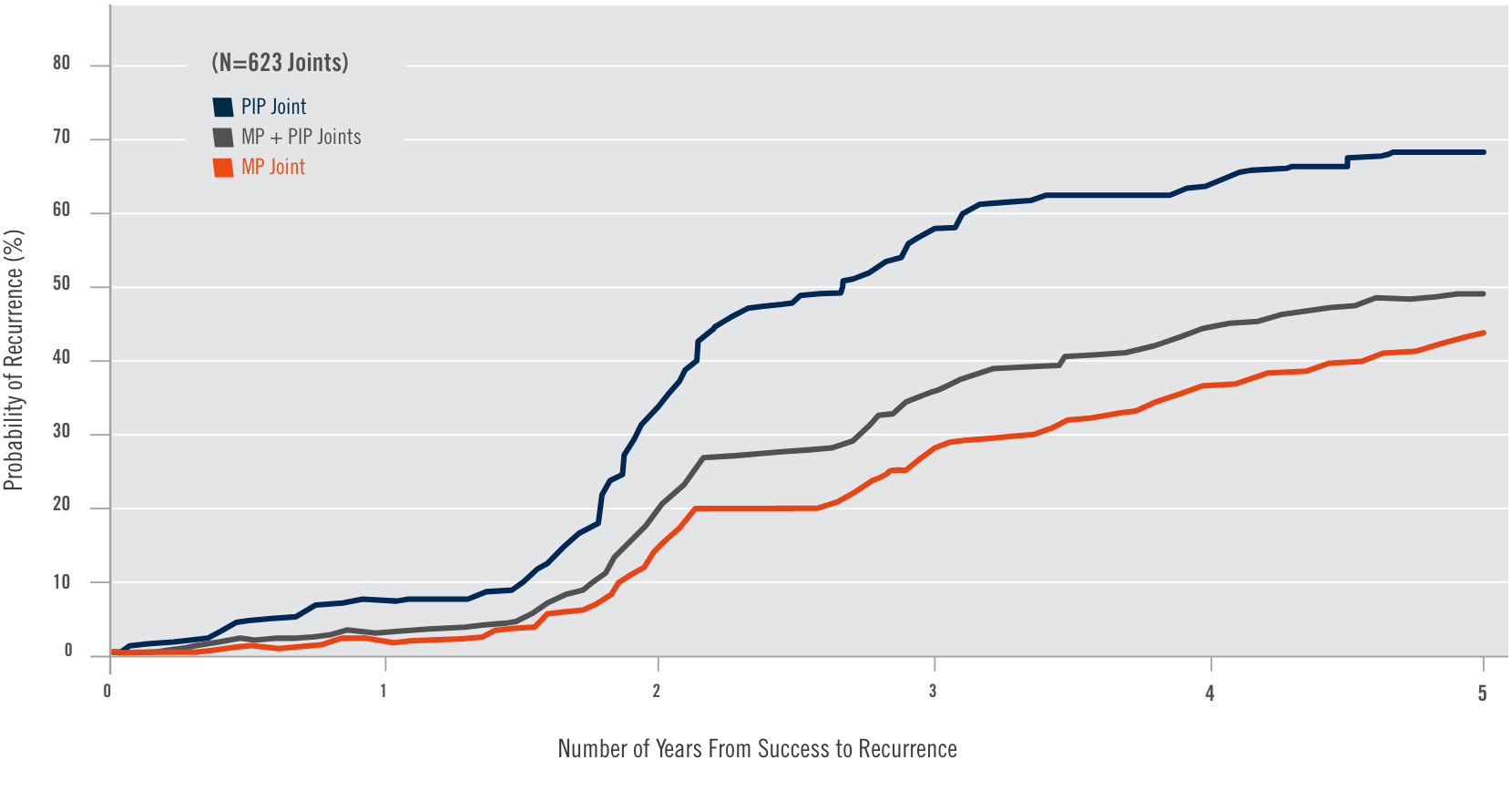
Percentage of patients achieving a reduction in contracture of the selected primary joint—MP or PIP—to within 0°—5° 30 days after the last injection of that joint on Days 30, 60, or 90 (after up to 3 injections)
Key inclusion criteria1
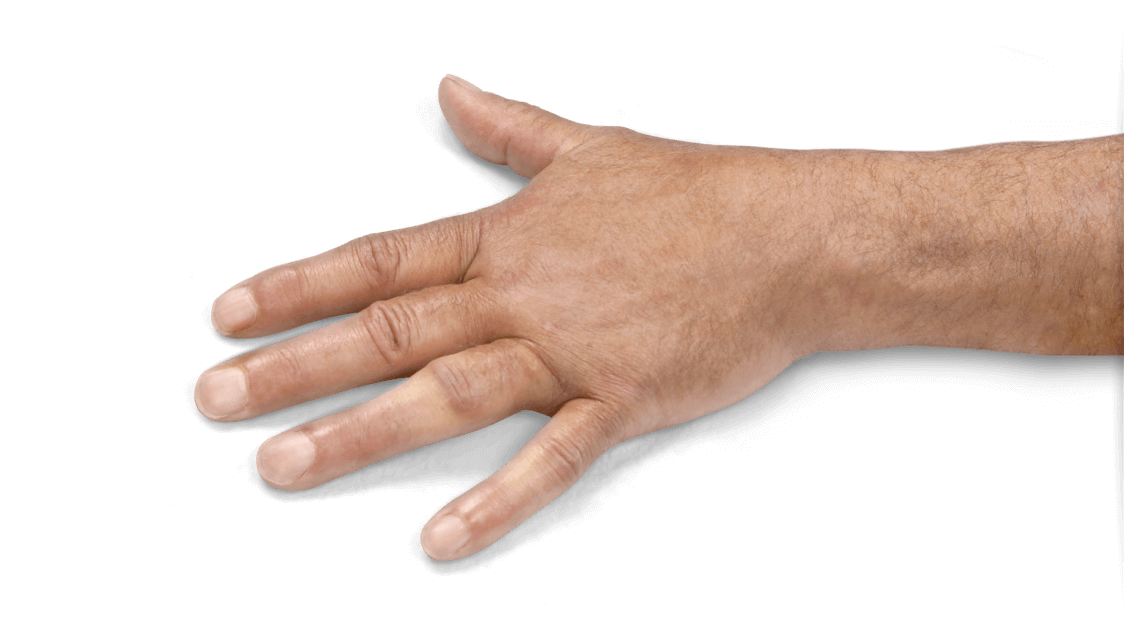
- Positive Tabletop Test
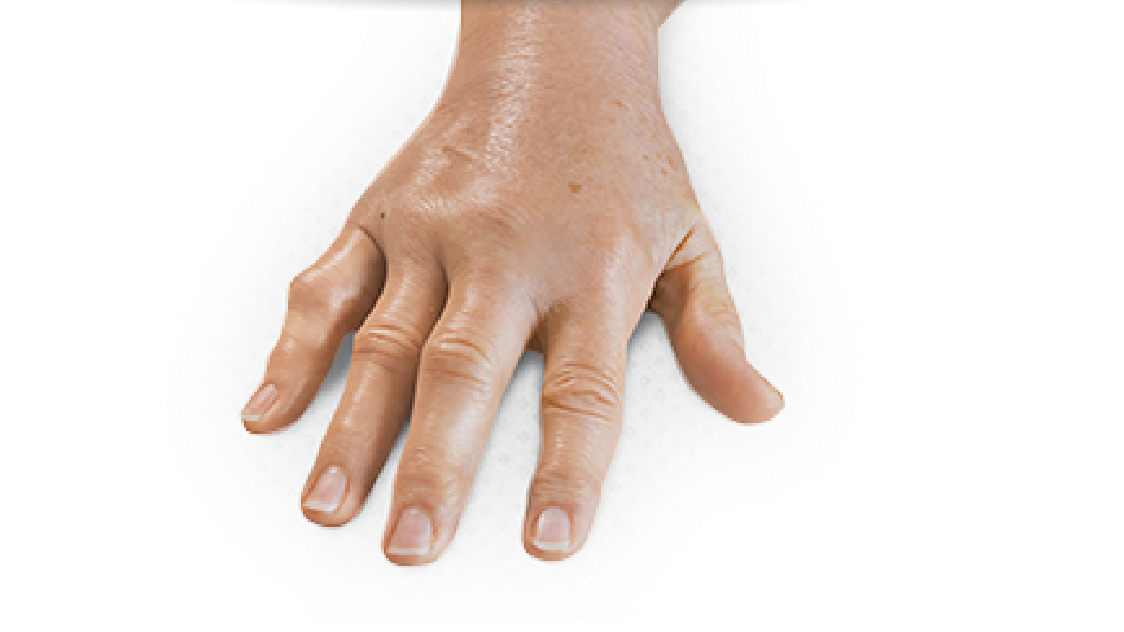
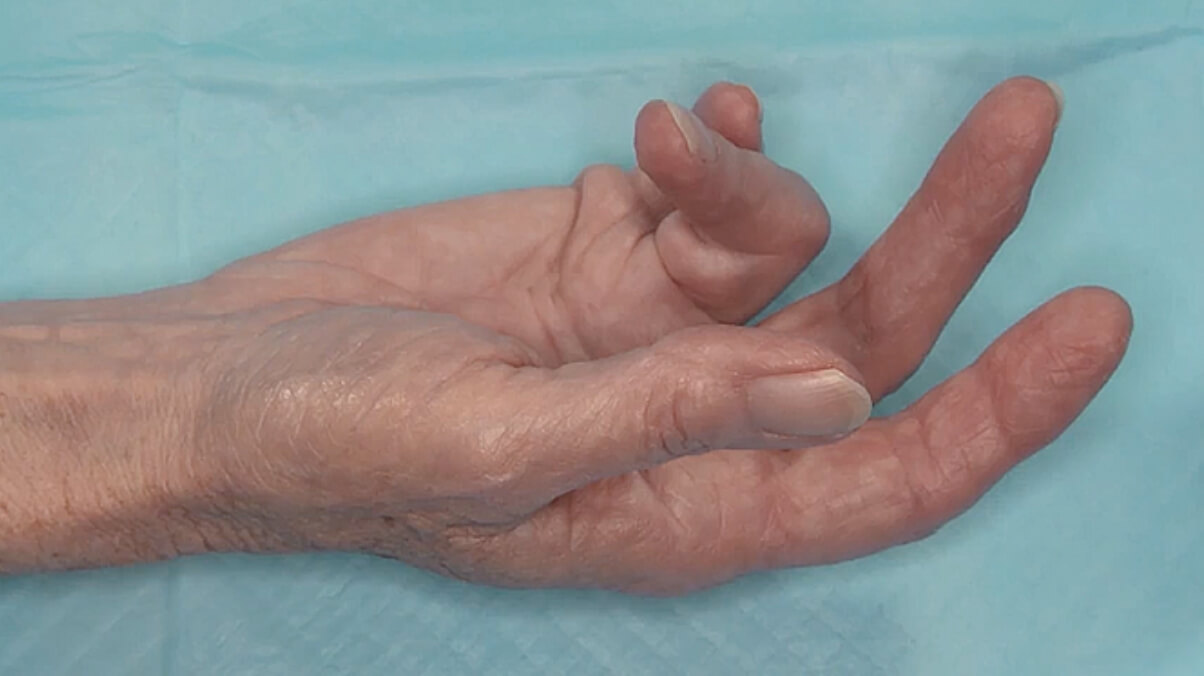
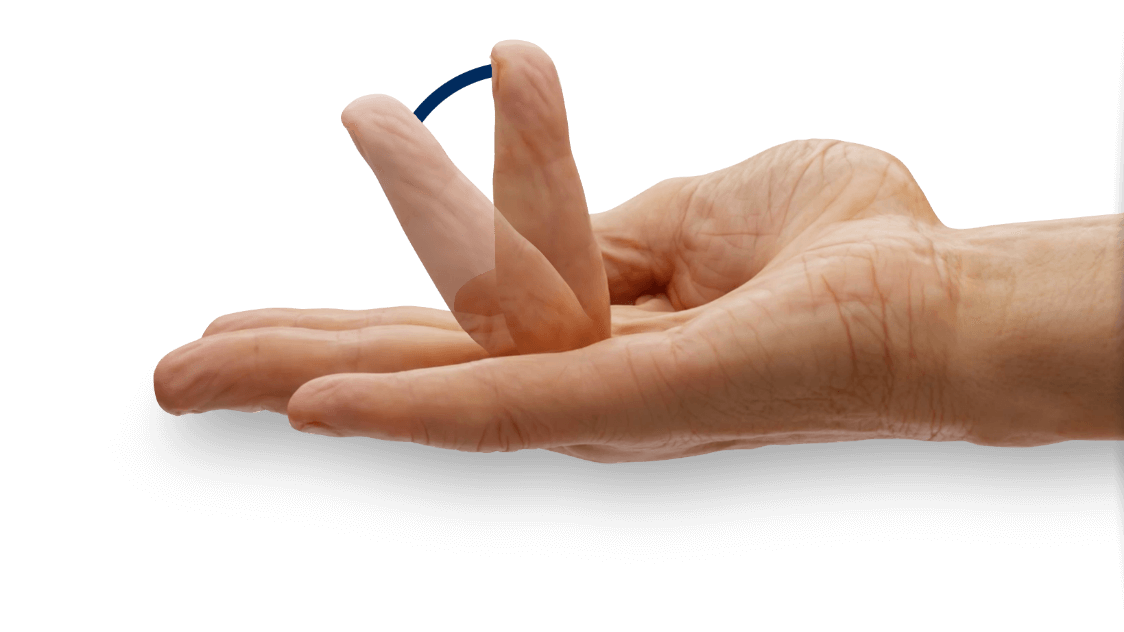
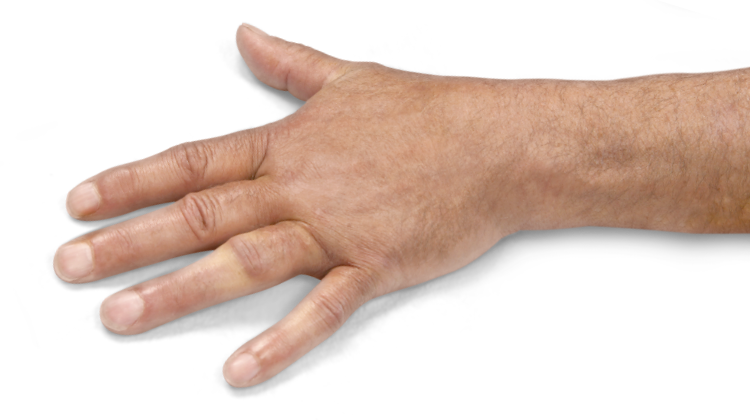
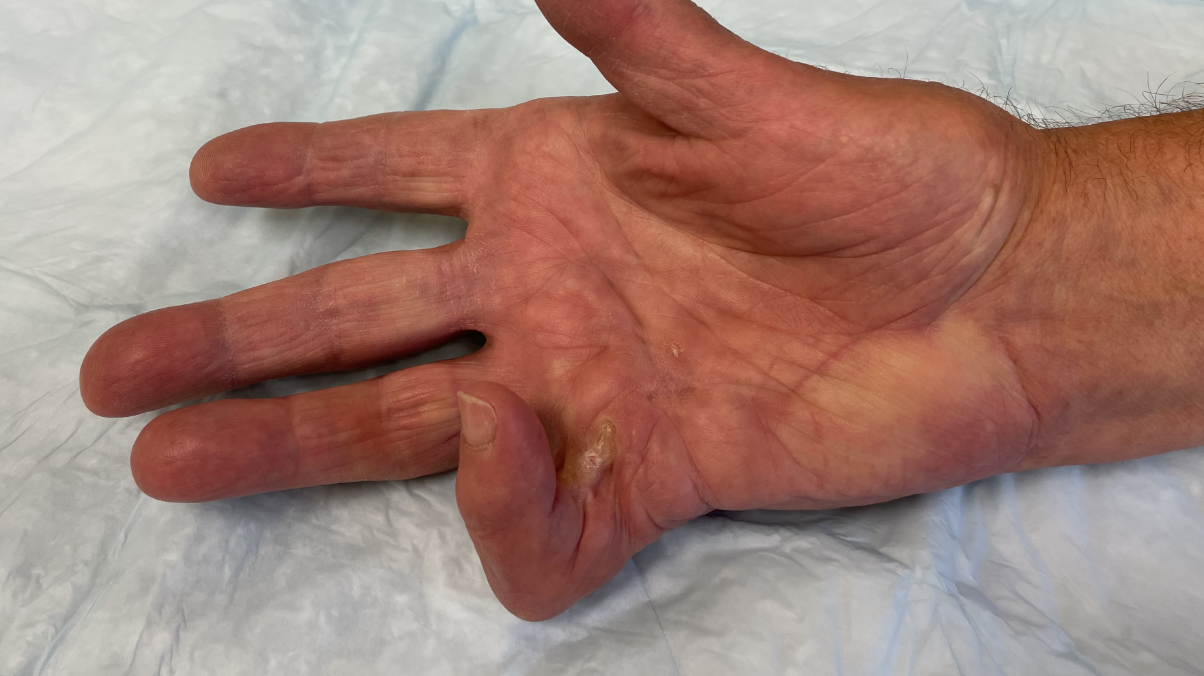
- Contracture of 1 finger (other than thumb) caused by palpable collagen-containing cord
- MP joint contracture: 20°—100°
- PIP joint contracture: 20°—80°
Patient Characteristics1
- Approximately 40% had been previously treated with surgery
- Had an average of 3 joints affected
Treatment
- The cord affecting the selected primary joint received up to 3 injections of 0.58 mg of XIAFLEX® or placebo on Days 0, 30, and 601
- About 24 hours after each injection of study medication, if needed, the investigator manipulated (extended) the treated finger in an attempt to facilitate rupture of the cord1
- Patients did not receive local anesthesia for the manipulation procedure during these trials2
- Following manipulation, patients were fitted with a splint, instructed to wear the splint at bedtime for up to 4 months, and instructed to perform a series of finger flexion and extension exercises each day1